Local colorectal surgeons with UBMD are participating in a clinical trial being done at Millard Fillmore Suburban Hospital (MFSH) utilizing a device that revolutionizes colorectal cancer surgery treatment, recovery, and quality of life for patients.
The device, Colorectal Balloon Tube, known as COLO-BT™ is a temporary intraluminal bypass device used following colorectal surgery. Developed by Dr. Kim and manufacturer JSR Medical Co., Ltd. in Korea, COLO-BT is being used by surgeons there and has received FDA approval for clinical trial use in the U.S. MFSH is one of only three hospitals selected for the trial.
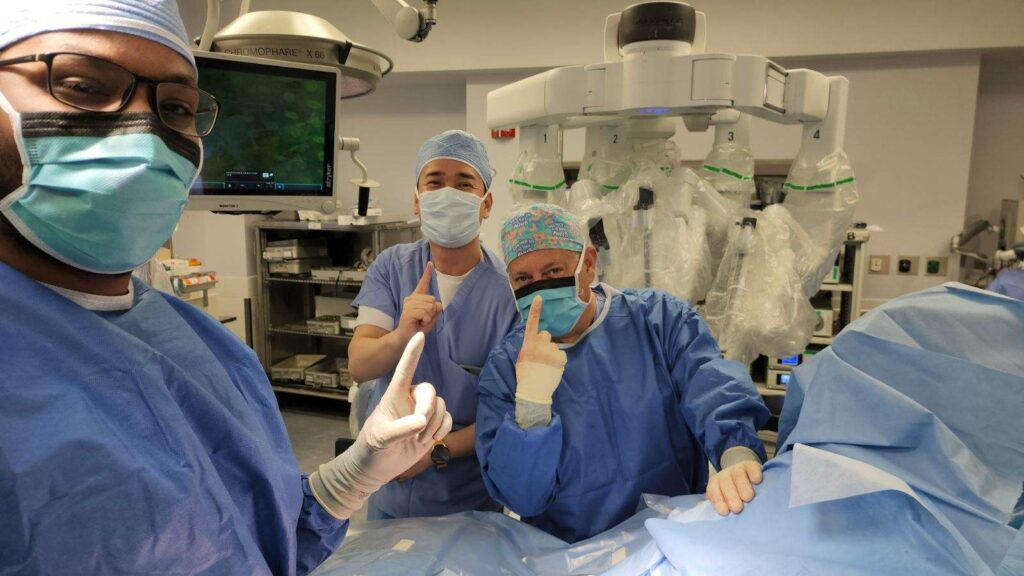
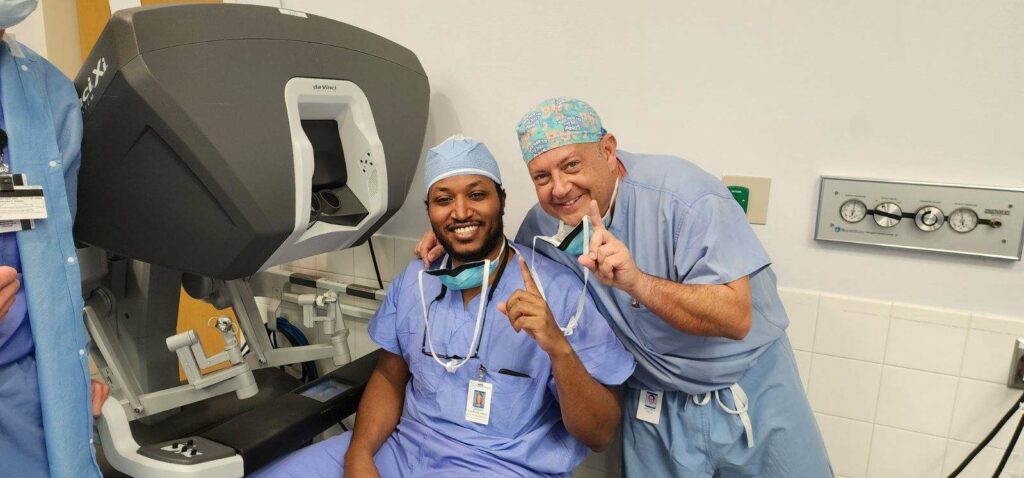
On Monday, the first patient in the U.S. underwent robotic-assisted colorectal cancer surgery and received implantation of the device here at MFSH.
COLO-BT is a medical device used to avoid the need for an ileostomy. An ileostomy is a surgical procedure that attaches part of the small intestine to an opening, or stoma, in the abdominal wall so waste can exit when the colon is not functioning properly. Most patients need a temporary ileostomy for 3-6 months following colorectal cancer surgery to allow the intestines to heal. Then the ileostomy is reversed requiring a second surgery. COLO-BT completely removes the need for an ileostomy and the second reversal surgery. The device significantly reduces the risk of complications associated with colorectal cancer surgery and those commonly developed afterward.
“This device brings life-changing advancement to the field of colorectal cancer surgery,” said Jeffrey Visco, MD, site medical director of Surgical Services at Millard Fillmore Suburban Hospital. “COLO-BT reduces complications that can occur with these complex surgeries and eliminates the patient having to wear an ostomy bag and undergo a second reversal surgery down the road. All significant improvements in patient care and safety speed up recovery and improve the quality of life for patients. We are very happy to be a part of this promising clinical trial,” said Visco.
The first recipient, Charles Jack, 53, a clinical psychologist, was recently diagnosed with rectal cancer requiring surgery. He was agreeable to the surgery and clinical trial with COLO-BT placement because it offers a less intrusive recovery process and a faster recovery. He underwent surgery Monday, March 4 with Dr. Visco and the surgical team at MFSH and one day later he is up and walking around in his hospital room independently. “I was considering living with the cancer because I did not want to deal with the surgeries and bag and all that goes with it,” said Jack. “Dr. Visco recommended the study and I thought it was a better option for me.”
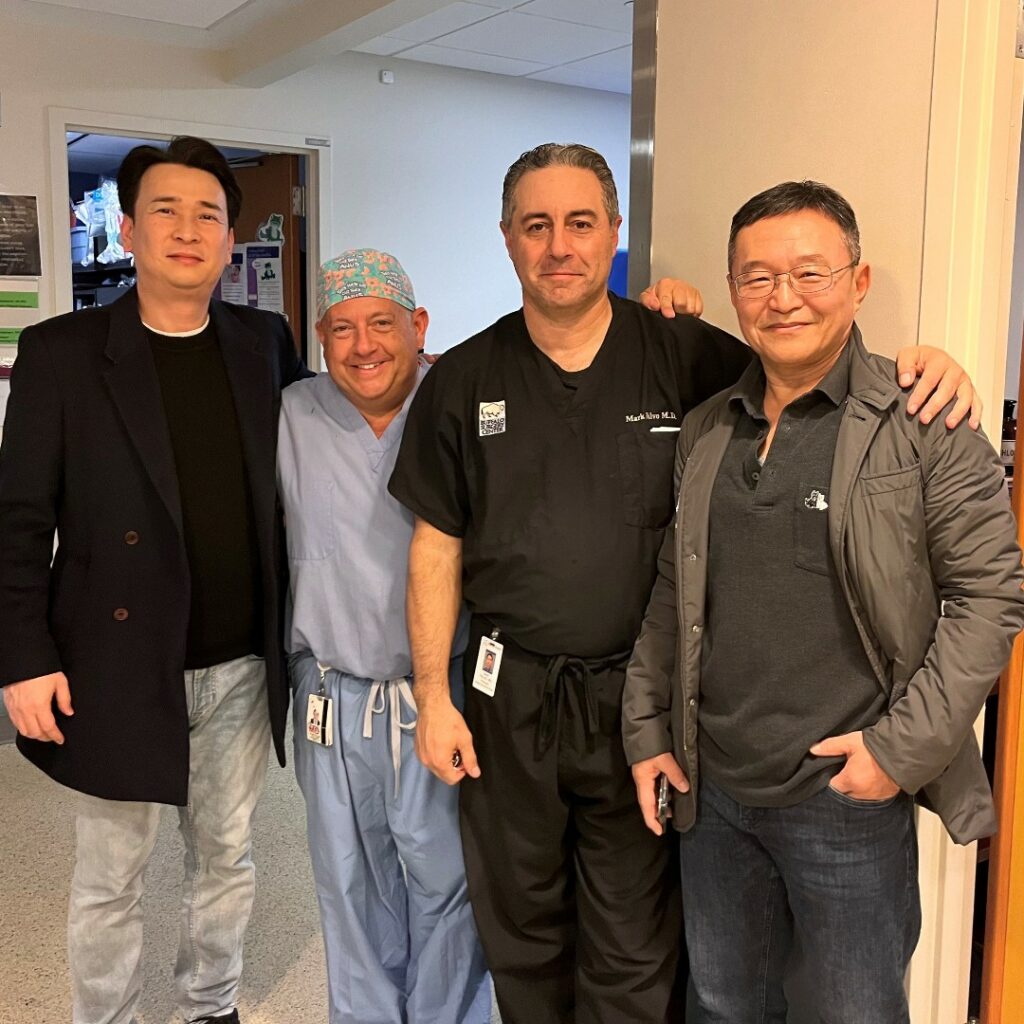
Founders from JSR Medical Inc., Dr. Kim, and Jake Kim, traveled 16 hours from Korea to be at the hospital for the first implantation in the country.
The COLO-BT study is set to allow 256 patients who meet specific criteria to participate in the clinical trial. Baylor and Penn State are also conducting the trial, however, UBMD surgeons at MFSH are serving as the lead investigators in the clinical study.